The 40 Year-old Discovery Behind A Promising New Flu Drug
Basic research led to a better antiviral drug to combat influenza

Robert Krug in his lab at Memorial Sloan-Kettering Cancer Center, circa 1970s.
A discovery that Robert Krug, a University of Texas at Austin molecular biologist, made decades ago has led to the development of a new drug to fight flu infections more effectively than existing drug treatments.
While the new drug, sold under the name Xofluza and already approved for use in Japan, has garnered a lot of media attention from outlets like Bloomberg, USA Today, Forbes and the Wall Street Journal, the focus has largely been on the drug's effectiveness and when it will be available here—potentially for next year's flu season.
The untold story is about the basic research, without which the drug would have been impossible, and how scientist Robert Krug made the discovery in the 1970s about how flu hijacks a critical process inside cells early on.
Existing flu treatments, such as Tamiflu, attack the virus after it has replicated in host cells; they work by making it harder for daughter viruses to escape from the host cell. The new drug Xofluza, on the other hand, attacks the flu virus at the early stage that Krug's research illuminated, blocking flu before it is able to create daughter viruses.
"It stops the virus cold," Krug said. "It can't do anything."
Theoretically, this should make the new drug exquisitely effective at halting progression of the disease. And already human trials have demonstrated that Xofluza patients have a much lower viral load in the first three days of treatment than Tamiflu patients and stop shedding flu virus in about a day, compared to about three days with Tamiflu.
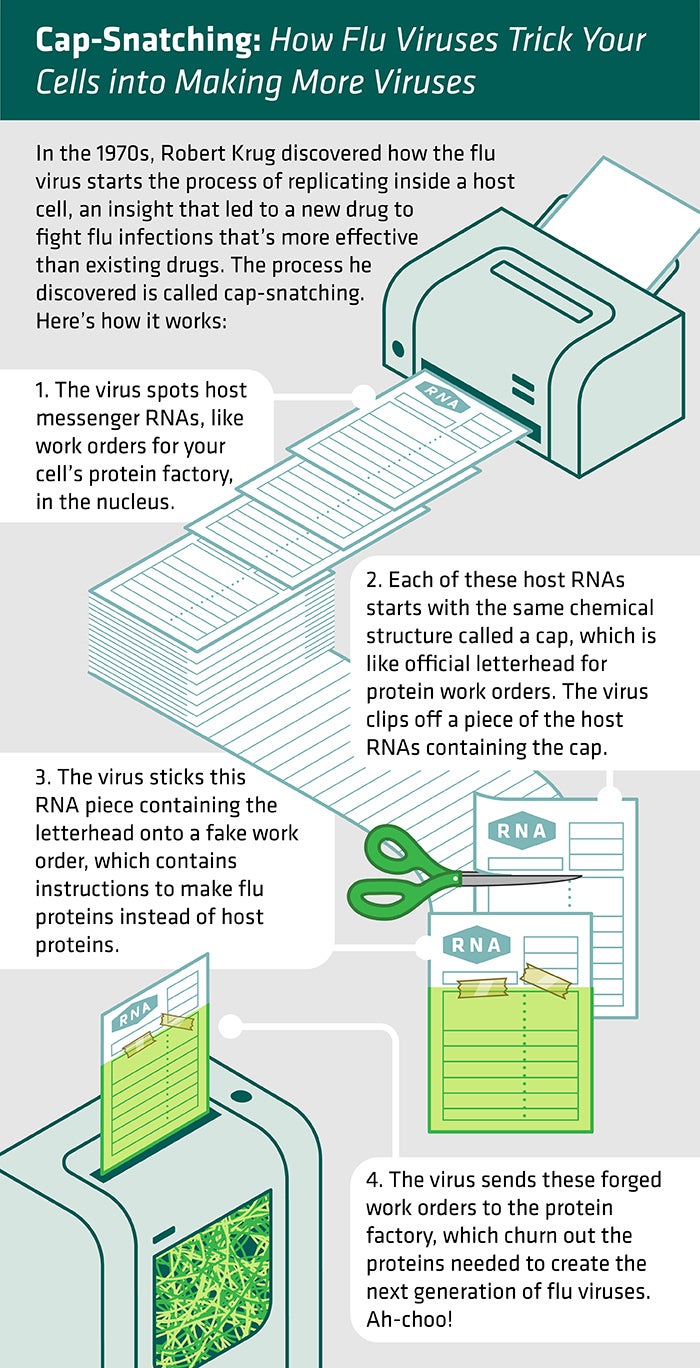
Illustration: Jenna Luecke
When Krug and his fellow flu researchers studied flu virus back in the 1970s, they knew that it tricks host cells into making viral proteins, the materials that are needed to form new copies of the virus so that it can spread. However, it was not known how the virus produced messenger RNAs, which carry the instructions for creating viral proteins.
Lab experiments showed that the virus couldn't initiate the process of making messenger RNAs on its own.
"If you took the virus out of a host cell and asked it to start making messenger RNAs, it simply couldn't do it," said Krug, who at the time was a researcher at the Memorial Sloan-Kettering Cancer Center.
So how did the virus start the process? Through some hard work, Krug and his team found out.
Normal cells in your body are constantly making proteins to build things, regulate the functions of life, communicate with other cells, and so on. A cell starts by making messenger RNAs, each with the same short chemical sequence on one end, called a cap. It's kind of like the official letterhead for work orders at the protein factory. Only messenger RNAs that start with that cap direct cells to make proteins.
In 1979, Krug and his team discovered that the ever-resourceful flu virus grabs an early-stage messenger RNA in the host cell, cuts off a piece of the host cell messenger RNA including the cap and sticks it onto its own RNA strand to make a viral messenger RNA that looks like ones that the host cell makes. And it works. The host cell takes these forged work orders and makes them into viral proteins. The researchers dubbed this pirating process cap-snatching.
"It's stealing a piece of a host cell messenger RNA to use as a primer to initiate the chains of viral messenger RNA," Krug said. "That was a big surprise. In fact, it was hard to publish the first paper. Nobody believed it."
Krug immediately wondered if a drug might disrupt this early step in viral replication and if so, would it be an effective weapon against flu infections? If you wanted to stop the flow of forged work orders, though, you had to find out a lot more about the cap-snatching part of a virus complex of protein molecules, called a polymerase.
It wasn't until 2014 that researchers at the European Molecular Biology Laboratory in France published research pinpointing this three-dimensional structure – a big, complicated molecule made of three proteins within the flu virus. This information provided the missing link that made Krug's discovery in the 1970s actionable. Soon after, Japan's drugmaker Shionogi discovered that a molecule known as baloxavir marboxil (now marketed as Xofluza) sticks to the viral polymerase and blocks its cap-snatching function.
Beyond being fast and effective, Xofluza is convenient. Treatment requires only one dose, whereas Tamiflu involves two pills a day for five days.
Already, medical researchers are taking notice. In Texas, for example, the Austin Regional Clinic is currently conducting a clinical trial of Xofluza among high-risk patients.
Researchers at the University of California, San Diego announced this week that they discovered another compound that inhibits the flu virus polymerase from carrying out cap-snatching, which they believe, based on chemical experiments, will be even more effective than Xofluza. They hope to begin testing it in humans in a couple of years.
Earlier this month, the Committee on Energy and Commerce of the U.S. House of Representatives held a hearing on treating flu with experts from the National Institutes of Health, Centers for Disease Control and Prevention and elsewhere. Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Disease, highlighted the importance of Krug's 1979 paper explaining the cap-snatching process. He noted that the work was funded by the National Institutes of Health.
"So it just goes to show you that basic science is the root of everything we do, even something that [40] years later turns into a product made by a Japanese company," Fauci said.
Krug said it's very gratifying to know that his research, regardless of how long ago, can help alleviate human suffering.
"I was just trying to solve a basic research problem," said Krug. "And when I solved that, it led to a new drug. It took 40 years, but still, I think it shows that you have to fund basic research because you just don't know when this research will turn out to benefit human health."



